During the nineteenth century the main theory in physics was still Newtonian Mechanics. With the advances in electricity, magnetism and the studies about light, it was clear that the theory had its limitations. The limitations appeared in the limits of the too small (atomic size) or the too fast (light speed).
The study of the small limit took a long path and it has several contributors. It started in the beginning of the 1900s and laid the foundation of the modern quantum and particle physics.
Max Plank and Albert Einstein proved that light has discrete particle properties, besides the already known wave's characteristics (the rainbow is its most natural example of the light seen as wave). The concept is known as duality wave-particle. The particle is known as the photon, and this theory can be observed in practice each time you turn on a florescent light. Albert Einstein received the Nobel Prize for this discovery (photoelectric effect) and not for the relativistic theory. Plank also received the Nobel Prize for his discoveries in this area.
Einstein Credits Max Plank
According to Albert Einstein, it was Max Plank who provided the single piece of information that ended up being quantum and particle physics. In his book On Quantum Physics written in 1954, he states:
"In the year nineteen hundred, in the course of purely theoretical (mathematical) investigation, Max Planck made a very remarkable discovery: the law of radiation of bodies as a function of temperature could not be derived solely from the Laws of Maxwellian electrodynamics. To arrive at results consistent with the relevant experiments, radiation of a given frequency f had to be treated as though it consisted of energy atoms (photons) of the individual energy hf, where h is Planck's universal constant."
Max Plank Credits Niels Bohr
Max Plank, in The Origin and Development of the Quantum Theory written in 1922 acknowledges Niels Bohr and his quantum theory of the atom the first step of quantum and particle theory:
"Although the results I have hitherto quoted from the most diverse chapters of physics, taken in their totality, form an overwhelming proof of the existence of the quantum of action, the quantum hypothesis received its strongest support from the theory of the structure of atoms (Quantum Theory of Spectra) proposed and developed by Niels Bohr. For it was the lot of this theory to find the long-sought key to the gates of the wonderland of spectroscopy which since the discovery of spectrum analysis up to our days had stubbornly refused to yield. And the way once clear, a stream of new knowledge poured in a sudden flood, not only over this entire field but into the adjacent territories of physics and chemistry. Its first brilliant success was the derivation of Balmer's formula for the spectrum series of hydrogen and helium, together with the reduction of the universal constant of Eydberg to known magnitudes (35); and even the small differences of the Eydberg constant for these two gases appeared as a necessary consequence of the slight wobbling of the massive atomic nucleus (accompanying the motion of electrons around it). As a sequel came the investigation of other series in the visual and especially the X-ray spectrum aided by Kitz's resourceful combination principle, which only now was recognized in its fundamental significance."
Bohr's model of the atom is taught even now in elementary school: it is the one that looks like the solar system in miniature. It might seem rather natural at this point, but it was not so natural when stated. Bohr came up with his model of the atom to explain why the collision experiments run to explore the nature of the atom showed big deflection angles. It also explained Balmer lines, which were very well known but not understood.
De Broglie and Schrodinger extended the concept of duality particle-wave to all material things, leading to discussions that where more philosophical that physicl. Schrodinger's equation is still the central equation to explain quantum phenomena.
Heisenberg, Bohr and Born introduced discrete statistical fields in replacement of the continuous deterministic fields predicted by Newton Mechanics. This fact explained for example why the electrons didn't collapse toward the nucleus of the atom and gave a first approximation to the localization of the particles.
Quantum and particle physics are two of the main branches of physics, they were developed by the common effort of hundreds of scientists and they are being still researched nowadays.


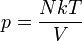
Weldon Vlasak
The above information is highly flawed. Whoever wrote it didn’t know how to spell “Planck”. He showed that black radiation comes from quantized jumps in energy hf. Bohr’s theory was abstract (by his own admission), in that the size of his atoms had no limit, although it does fit the Rydberg frequencies of radiation fairly well. It took some 70 years before physicists admitted to this fact.