What are Alpha Particles?
An alpha particle is a type of ionizing radiation that is exerted from radioactive materials. Alpha particles consist of two neutrons, two protons, and no electrons, giving it a net positive charge. This positive charge allows the alpha particles to leave their nucleus and bombard any nearby items with radiation. Alpha particles have a high mass, which makes them the most destructive form of radiation compared to others such as gamma particles and beta particles, which are much lighter.
Where Do Alpha Particles Come From?
Alpha particles come from a number of sources including stars and radioactive materials such as americium, radon gas, radium, uranium, and polonium. These materials may be found naturally in food, air, and water but are also often generated through artificial means such as x-rays, smoke detectors, and ionization chambers. Alpha particles are emitted from a substance when internal changes cause the nucleus to lose two of its protons and two of its neutrons. An improper ratio of neutrons to protons most often causes this. When an alpha particle is emitted from a substance, the substance actually becomes a different element altogether because each element has a specific number of protons.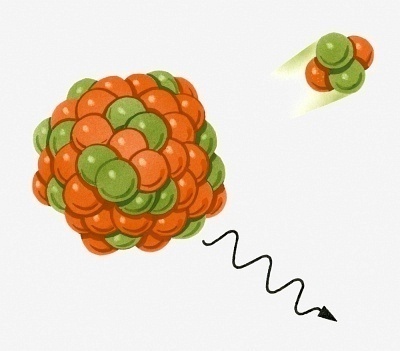
Nuclear Reactions
Alpha particles are essential in nuclear reactions because they are used to agitate neutron emitters. Neutron emitters are basically the same as alpha emitters but they emit neutrons rather than protons. By mixing substances such as uranium and plutonium a nuclear reaction can occur. While neutron emitters can be agitated by other methods and do not require alpha particles, alpha emitters make the nuclear process much smoother and allow it to be carried out in a controlled environment. Alpha particles are a crucial part of modern nuclear reactors and nuclear weapons.
Health Effects
While alpha particles have a high mass and are the most destructive form of radiation, they also have a low penetrating force. This weak penetration causes alpha particles to be stopped by most objects, even a simple sheet of paper. This is very different from other forms of radiation that only stronger materials such as aluminum can block. In fact, alpha particles have such a weak penetration that they cannot even pass through human skin. This means that alpha particles are rarely ever dangerous to humans and can only be lethal when the decaying material is ingested. However, it should be noted that tobacco leaves contain small traces of polonium, which may be responsible for the carcinogenic effects of smoking tobacco.
Evolution
Though alpha particles cause over 100 times more chromosomal mutations than other forms of radiation, it is ironic that these mutations are actually what cause evolution. Alpha emitters in nature cause the chromosomes within humans and animals to mutate and usually result in undesirable effects. However, it can cause biological adaptation over the course of thousands or millions of years.



Follow Us!